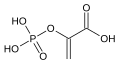
Phosphoenolpyruvate carboxykinase
Phosphoenolpyruvate carboxylase (or PEPCase) is an enzyme in the family of carboxy-lyases. While most reactions of gluconeogenesis simply use the glycolysis enzymes in the opposite direction, the pyruvate kinase enzyme is irreversible. Therefore, the enzymes pyruvate carboxylase and phosphoenolpyruvate carboxykinase are used to provide an alternate path for effectively reversing its actions.
PEPCK gene transcription (genetics) occurs in many organisms. The amino acid sequence of PEPCK is distinct to the organism. For example, its structure and its specificity differ in humans, Escherichia coli (E. coli), and the parasite Trypanosoma cruzi (Trapani et al., 2001). In mammals, it is most abundant in the liver, kidney, and adipose tissue (Chakravarty et al., 2005). In an effort to explore the role of PEPCK, researchers caused the overexpression of PEPCK in E. coli bacteria via recombinant DNA (Aich et al., 2003). It has been shown that PEPCK catalyzes the reversible rate-controlling step of gluconeogenesis, the process whereby glucose is synthesized. The enzyme has therefore been thought to be essential in glucose homeostasis, as evidenced by laboratory mice that contracted diabetes mellitus type 2 as a result of the overexpression of PEPCK (Vanderbilt Medical Center). Recent research, however, demonstrated that PEPCK may not play as direct a role in gluconeogenesis as was previously believed, as PEPCK levels were more highly correlated with citric acid cycle activity than with gluconeogenesis in the mouse liver (Burgess et al., 2007). PEPCK of Mycobacterium tuberculosis has been shown to trigger the immune system in mice by increasing cytokine activity (Liu et al., 2006). As a result, it has been found that PEPCK may be an appropriate ingredient in the development of an effective subunit vaccination for tuberculosis (Liu et al, 2006).
PEPCK in Plants and Bacteria
PEPCK acts in the most biochemically complicated C4 photosynthetic plants, where its action has been localized to the cytosol, in contrast to mammals, where it has been found that PEPCK works in mitochondria (Voznesenskaya et al., 2006). Although it is found in many different parts of plants, it has only been seen in specific cell types, including the areas of the phloem (Chen et al., 2004). It has also been discovered that in cucumber (Cucumis sativus L.), PEPCK levels are increased by multiple effects that are known to decrease the cellular pH of plants, although these effects are specific to the part of the plant (Chen et al, 2004). PEPCK levels rose in roots and stems when the plants were watered with ammonium chloride at a low pH (but not at high pH), or with butyric acid. However, PEPCK levels did not increase in leaves under these conditions. In leaves, 5% CO2 content in the atmosphere lead to higher PEPCK abundance (Chen et al, 2004).
Structure
The structures formed when PEPCK complexes with other substances provide insight into the structure and also the mechanism of PEPCK enzymatic activity (Holyoak et al., 2006). The three mitochondrial isoforms of PEPCK complex with Mn2+, Mn2-PEP, and Mn2-malonate- Mn2 + GDP to give more information about its structure and how this enzyme catalyzes reactions (Holyoak et al., 2006). Delbaere et al. (2004) resolved PEPCK in E. coli and found the active site sitting between a C terminal domain and an N terminal domain. The active site was observed to be closed upon rotation of these domains (Delbaere et al., 2004). Phosphoryl groups are transferred during PEPCK action, which is likely facilitated by the eclipsed conformation of the phosphoryl groups when ATP is bound to PEPCK (Delbaere et al, 2004). Since the eclipsed formation is one that is high in energy, phosphoryl group transfer has a decreased energy of activation, meaning that the groups will transfer more readily. This transfer likely happens via a mechanism similar to SN2 displacement (Delbaere et al, 2004).
Reaction Pathway
PEPCase converts oxaloacetate into phosphoenolpyruvate, and carbon dioxide.
As PEPCK acts at the junction between glycolysis and the Kreb’s cycle, it causes decarboxylation of a C4 molecule, creating a C3 molecule. As the first committed step in gluconeogenesis, PEPCK decarboxylates and phosphorylates oxaloacetate (OAA) for its conversion to PEP, when GTP is present. As a phosphate is transferred, the reaction results in a GDP molecule (Holyoak et al, 2006). Interestingly, when pyruvate kinase, the enzyme that normally catalyzes the reaction that converts PEP to pyruvate, is knocked out in mutants of Bacillus subtilis, PEPCK participates in a replacement anaplerotic reaction, working in the reverse direction of its normal function, converting PEP to OAA (Zamboni et al., 2004). Although this reaction is possible, the kinetics are so unfavorable that the mutants grow at a very slow pace or do not grow at all (Zamboni et al., 2004). In fermentation, PEPCK catalyzes the reaction of PEP and carbon dioxide to OAA and ADP is therefore converted to ATP with the addition of a phosphate group (Chao et al, 1994).
Regulation
PEPCK is enhanced, both in terms of its production and activation, by many factors. Transcription of the PEPCK gene is stimulated by glucagon, glucocorticoids, retinoic acid, and adenosine 3’,5’-monophosphate (cAMP), while it is inhibited by insulin (O’Brien et al., 1990). Of these factors, insulin, a hormone that is deficient in the case of diabetes, is considered dominant, as it inhibits the transcription of many of the stimulatory elements (O’Brien et al, 1990). PEPCK activity is also inhibited by hydrazine sulfate, and the inhibition therefore decreases the rate of gluconeogenesis (Mazzio & Soliman, 2003). As discussed previously, PEPCK abundance increased when plants were watered with low pH ammonium chloride, though high pH did not have this effect. The GTP-specific activity of PEPCK is highest when Mn2+ and Mg2+ are available (Aich et al., 2003). In addition, hyper-reactive cysteine (C307) is involved in the binding of Mn2+ to the active site (Holyoak et al., 2006).
Classification
It is classified under EC number 4.1.1. There are three main types, distinguished by the source of the energy to drive the reaction:
- 4.1.1.32 - GTP (PCK1, PCK2)
- 4.1.1.38 - diphosphate
- 4.1.1.49 - ATP
External links
- Phosphoenolpyruvate+Carboxykinase+(ATP) at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Phosphoenolpyruvate+Carboxykinase+(GTP) at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- . GPnotebook https://www.gpnotebook.co.uk/simplepage.cfm?ID=-872021957.
{{cite web}}: Missing or empty|title=(help)